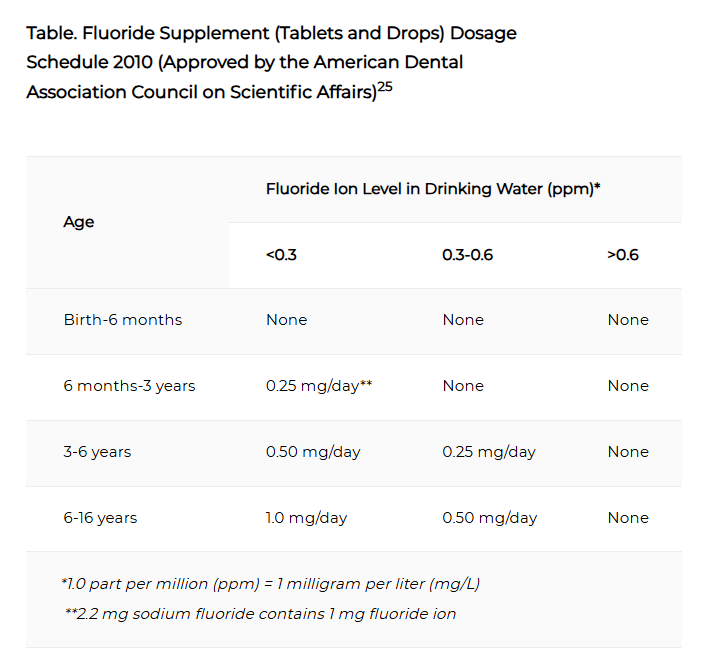
Fluoride Supplement Recommendations
The use of dietary fluoride supplements is one alternative means of providing protection to the teeth of children six months old to 16 years of age who consume fluoride-deficient water with 0.6 ppm fluoride or less. Dietary fluoride supplements, in the form of daily tablets, drops or vitamin-fluoride combinations, provide systemic benefits to developing teeth as well as topical benefits to erupted teeth.
When prescribed and used appropriately, fluoride supplements provide benefits similar to those obtained from ingesting optimally fluoridated water over the same period of time. When improperly prescribed, fluoride supplements may cause enamel fluorosis. Therefore, systemic fluoride supplements should never be prescribed to children in fluoridated communities who are receiving optimally fluoridated water (0.7 ppm fluoride).
Because of an increase in the milder forms of dental fluorosis associated with fluoride ingestion in excess of that necessary to prevent tooth decay, a conservative approach to fluoride supplementation should be used and be in accordance with the revised guidelines listed below. If a child's primary drinking water source is a well, spring or non-fluoridated community water system, a water sample must first be taken and analyzed to determine the fluoride content and the dosage of fluoride supplement needed, if any.