COVID-19 Vaccine Information Page
There are many COVID-19 vaccines currently in development and in clinical trials. The availability of a safe and effective COVID-19 vaccine depends on the outcomes of the Phase 3 clinical trials that are currently underway. If and when a vaccine is shown to be safe and effective in the Phase 3 clinical trials, it will be issued authorization by the Food and Drug Administration (FDA) for use in a limited number of individuals who are at high-risk for developing COVID-19 or who are at high risk of serious complications from COVID-19. The vaccine will continue to be monitored as it is used in larger populations to ensure that any indication of rare complications from its use are detected as soon as possible and evaluated to see if they were caused by the vaccine.
The Tennessee Department of Health (TDH) is working to ensure that a vaccine can be allocated and distributed to priority populations once that is made official. TDH will continue to monitor and share information about the COVID-19 vaccine on this webpage as it becomes available.
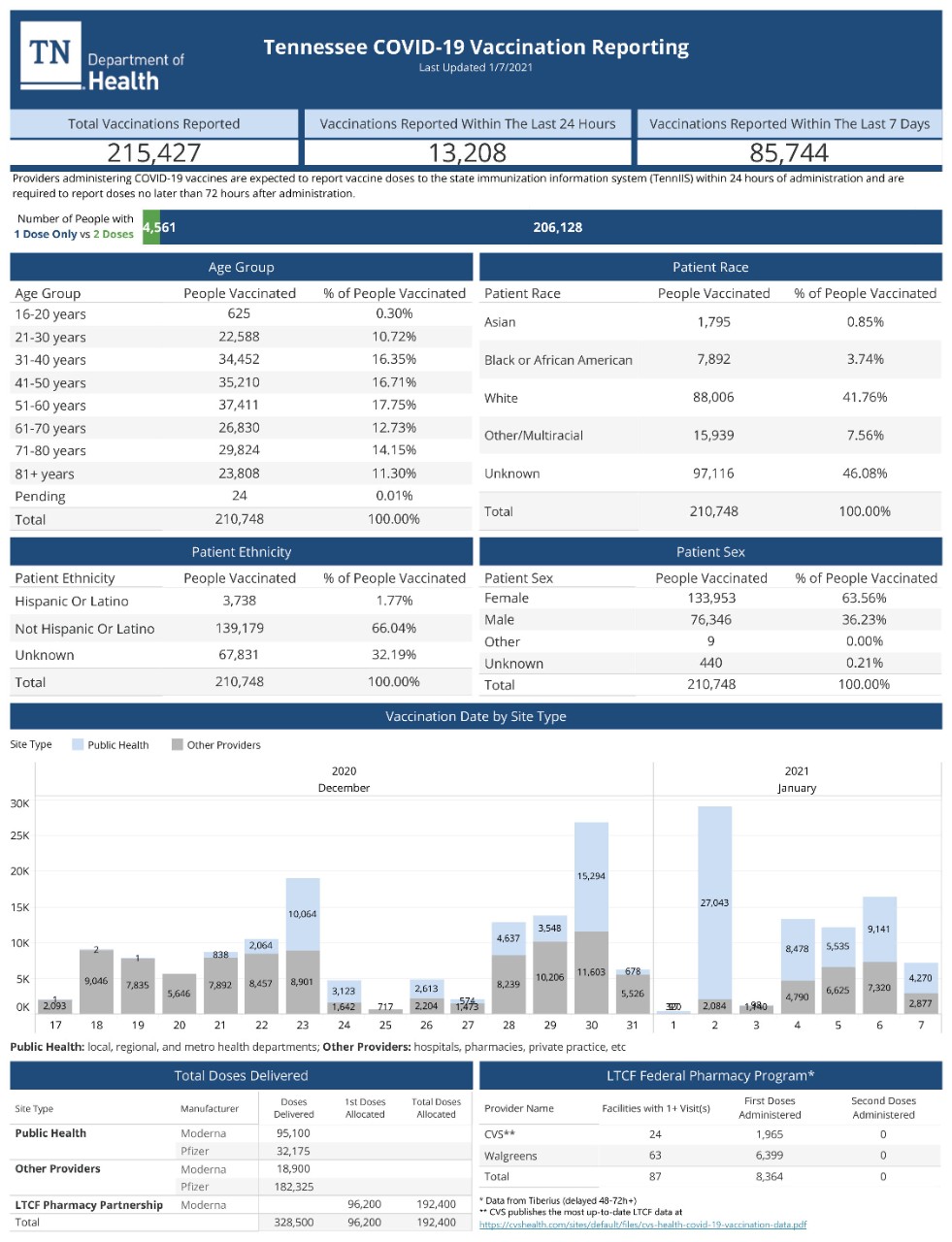
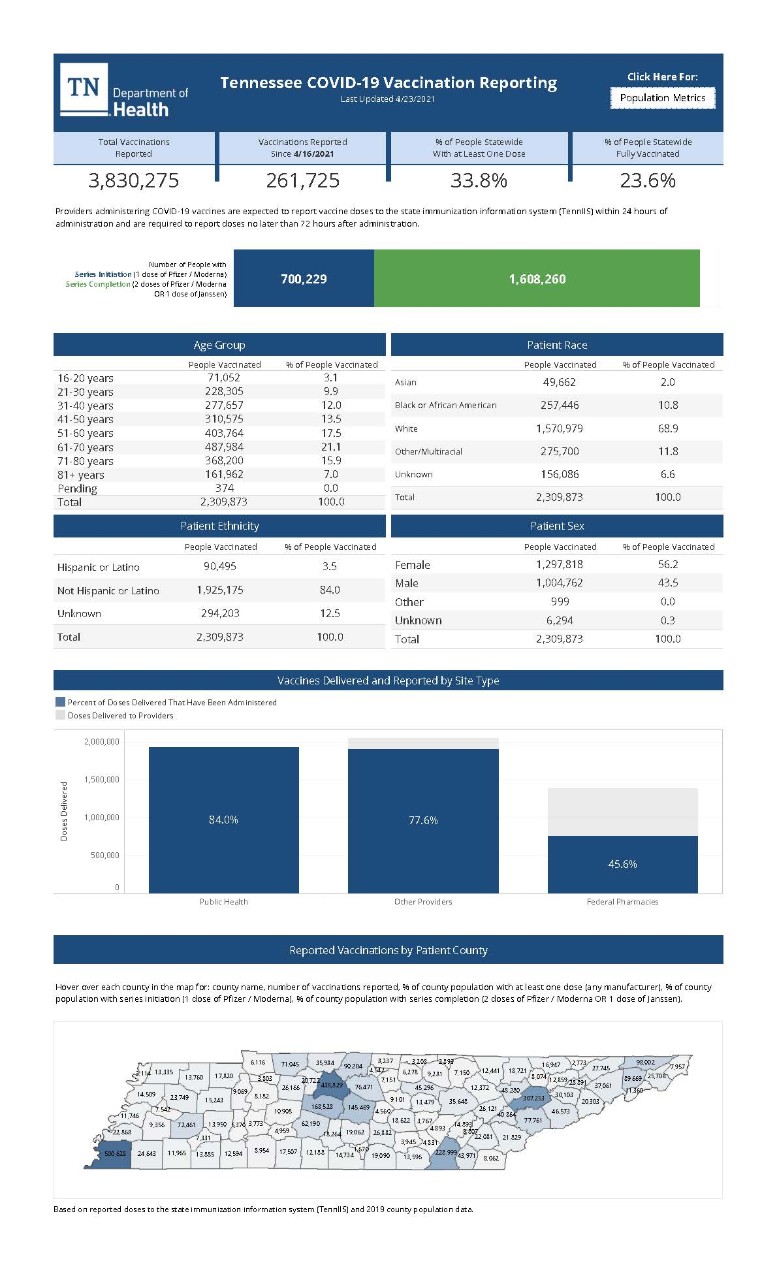
COVID-19 Vaccine Specific Information
- CDC COVID-19 Vaccine Information
- Pfizer-BioNTech COVID-19 EUA Recipient/Caregiver Fact Sheet
- Moderna Vaccine - What to Expect
- Emergency Use Authorization Table
- Getting the Second Dose (12/3) | Spanish (12/15)
- Vaccine Adverse Event Reporting System
- V-safe Information Sheet | Spanish | Chinese | Vietnamese | Korean


