Mercury
Should a mercury-containing thermometer or light bulb break, do not reach for the vacuum cleaner to clean up the mercury. Instead, open a window to ventilate the area. Then read our fact sheet describing proper cleanup procedures for small mercury spills.
Introduction to mercury
Mercury occurs naturally in the environment and exists in several forms. These forms can be organized under three headings: metallic mercury (also known as elemental mercury), inorganic mercury and organic mercury. Metallic mercury is the kind found in thermometers and some light bulbs. It is a silver-colored liquid at room temperature. It easily evaporates. Breathing large amounts of mercury vapors can cause harm. Touching or even swallowing metallic mercury is not very harmful. Although uncommon, inorganic mercury is toxic to the human body. In large amounts, organic mercury found in fish and wildlife can potentially be harmful.
Mercury thermometers
Old thermometers may contain mercury. If broken open, the spilled liquid mercury can be messy. A small mercury spill, such as a broken thermometer, is a nuisance but not an emergency. The amount of mercury in a thermometer is so small that it is not believed to be a health hazard. For small spills, do NOT use a vacuum cleaner. You can push the beads of mercury together with an piece of cardstock. Or use tape or an eye dropper to pick up the liquid mercury. Always ventilate the area well for many hours to replace an mercury vapors with fresh air. If a broken thermometer is cleaned up properly and promptly, your family will be safe.
Mercury was used for many years in thermometers designed for household use because no alternatives were available. However, this is no longer the case. In July 2001, the American Academy of Pediatrics issued a policy statement about the health effects of mercury. They also urged doctors and parents to stop using mercury thermometers and to dispose of them properly. Today, better alternatives such as digital or mercury-free thermometers are readily available.
Proper disposal
Mercury thermometers, as well as other types of mercury-containing items such as old thermostats, barometers, meat thermometers, candy thermometers, manometers, blood pressure cuffs, and other household mercury waste or devices, can be brought to a Household Hazardous Waste Collection where they will be accepted for proper disposal. Municipalities such as Davidson, Hamilton, Knox and Shelby counties have permanent sites. Other counties may have special collection days. For more information about how to properly dispose of household hazardous waste in Tennessee, please visit or call 615-532-9265.
Light bulbs
There are several types of light bulbs that contain mercury. Fluorescent tubes, metal halide, mercury vapor and compact fluorescent light bulbs (CFLs) all contain various amounts of mercury. The amount of mercury in a light bulb is far less than what is contained in a thermometer. Much of the mercury in a light bulb is a vapor or a dust. Remember to responsibly dispose of light bulbs in an environmentally safe manner.
Some people have asked, “How can compact fluorescent bulbs (CFLs) be more environmentally friendly if they contain mercury?” The answer is that CFLs are highly energy efficient. A typical incandescent light bulb burns hot. Most of the energy used by the light bulb is released as heat. Only about a small amount of the energy used is released as light. CFLs are the opposite, producing lots of light and only a little heat. CFLs use 50-80% less energy than old fashioned light bulbs. To make electricity, fossil fuels such as coal are often burned. When the electricity is generated, mercury is a by-product. Less energy to power CFLs mean less mercury by-product. Over the lifetime of an energy-efficient CFL, there is less mercury created as a by-product of the electricity generated than the tiny amount of mercury present inside the CFL.
Chemistry of mercury
Mercury occurs naturally in the environment and exists in several forms. These forms can be organized under three headings: metallic mercury (also known as elemental mercury), inorganic mercury and organic mercury. Metallic mercury is the kind of mercury found in thermometers. Metallic mercury is a silver-colored liquid at room temperature. It can easily evaporate. Breathing large amounts of mercury can cause harm. Most inorganic mercury compounds are white powders or crystals, except for mercuric sulfide (also known as cinnabar) which is red, but turns black after exposure to light. Inorganic mercury compounds occur when mercury combines with elements such as chlorine, sulfur, or oxygen. These mercury compounds are also called mercury salts. Mercury salts can cause toxic effects if they are ingested. Organic mercury compounds, also known as organomercurials, are formed when mercury combines with carbon. The most common organic mercury compound in the environment is methylmercury which bioaccumulates in fish and can be harmful if eaten.
Mercury cycles
Mercury cycles in the environment as a result of natural and human activities. The graphics below show two ways that mercury cycles through the environment. The first graphic shows how mercury emissions from human activities such as fossil fuel burning, waste incineration, or accidental releases move by weather and wildlife throughout our environment.
Improper disposal of mercury can contaminate water bodies. Natural processes can convert mercury in sediments into organic methylmercury. The methylmercury can be stored in the bodies of fish and wildlife. This type of mercury can be harmful if large amounts are ingested.
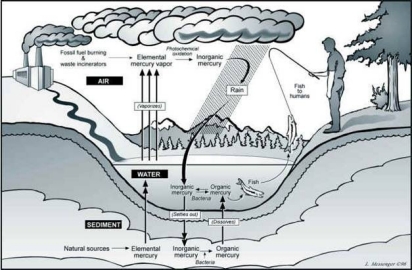
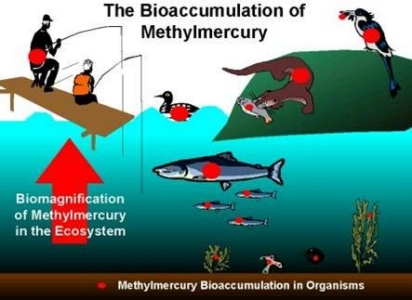
The second graphic shows how organic mercury can build up in living organisms. The graphic shows how methylmercury in lake sediments can get into fish. As the fish are eaten by larger animals, including humans, the methylmercury is passed along. Over time, this methylmercury can bioaccumulate in these larger animals. Eating large amounts of contaminated fish or wildlife can be a health hazard to people. In general, the larger predatory fish bioaccumulate more methylmercury than smaller pan fish. In small amounts, the health benefit of eating a balanced diet that includes fish is believed to be beneficial even though a small amount of methylmercury maybe ingested.
Phone numbers
You can clean up small amounts of mercury on your own. If you have questions or need advice, you may call us at the Tennessee Department of Health’s Communicable and Environmental Disease Services at 1-800-741-7247. The Tennessee Department of Environment and Conservation’s environmental hotline number is 1-888-891-TDEC (8332).
For a large mercury spill, your area’s Hazardous Materials (HazMat) unit, often located in the Fire Department, may be able to assist.
Additional resources
Tennesssee Department of Health
Healthy Homes
https://www.tn.gov/health/cedep/environmental/healthy-homes.html
Agency for Toxic Substances and Disease Registry (ATSDR)
Mercury in Schools page
https://www.atsdr.cdc.gov/dontmesswithmercury/mercury_school.html
U.S. Environmental Protection Agency (EPA)
Mercury
www.epa.gov/mercury
American Association of Pediatrics
https://www.aap.org/en-us/advocacy-and-policy/federal-advocacy/Pages/EnvironmentalHealth.aspx
U.S. Geological Society (USGS)
Mercury Research
http://minerals.usgs.gov/mercury
Centers for Disease Control and Prevention (CDC)
Mercury in Vaccines
http://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html
Health Canada
It's Your Health
http://www.hc-sc.gc.ca/hl-vs/iyh-vsv/environ/merc-eng.php
South Florida Restoration Science Forum
http://sofia.usgs.gov/sfrsf/rooms/mercury/food_chain
